Lately, I've been refactoring some code for a software engineering project completed earlier this year and for which additional work is expected. As a result, I've come to the conclusion that doing occasional refactoring is an absolute must for any programmer. I...
Month: July 2013
PLM in the Cloud: Computer System Validation in FDA Regulated Industries
Product lifecycle management (PLM) systems have evolved from being custom-built, on-premise applications to cloud-based, off-the-shelf solutions. As adoption for PLM in the cloud increases, system validation approaches in FDA/GXP regulated industries have had to...
3 Must-Have Mods for Windows 8
New software may not be something you want to use or look at, but if you are in the IT services field, it is necessary. Windows 8 has been a controversial release with many people refusing to upgrade and use it, stating that it is a mistake by Microsoft. Despite...
ROHS 2 for Medical Devices: Are You Ready?
As of July 22, 2014, the RoHS (Restriction of Hazardous Substances) Directive must be observed for first time distribution of all medical devices to the full extent. Furthermore, all products with a CE marking must also be RoHS-compliant. ROHS 2 Compliance Changes at...
Automatically Scraping Webpages using Python 2.7
In today’s Internet, it takes specific skills to efficiently find the “Data You Want” inside of the “Data You’re Given”. I was reminded of this the other day watching a colleague struggle through data collection, clicking buttons and getting diverted by advertisements...
Configuring a Build Job Using Hudson
Last time we had a look at how to acquire and install Hudson, a continuous build and integration system beneficial to software engineering. This week we take a look at how to create and run a job using Hudson, and then look at the job output. Hudson can run a wide...
The FDA UDI Rule: 5 Things You Need to Know
The release of the FDA final rule on Unique Device Identification (UDI) is expected this summer. Here are five things you need to know: 1. What is the UDI Rule? In July 2012, the FDA proposed a rule requiring medical device manufacturers to label their products with...
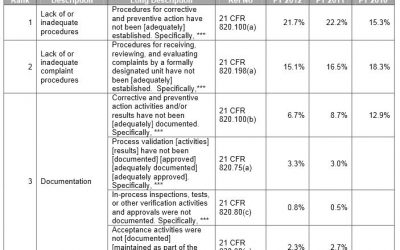
FDA Form 483: Top Ten Observations for Medical Devices
Medical Device manufacturers regulated by the FDA are subject to cGMP (Current Good Manufacturing Practice) regulations and may be inspected by the FDA to ensure compliance. If the FDA inspector(s) observes conditions that in their judgment may constitute violations,...
Tortoise for Windows — a Better Software Management Option
As part of our software engineering services, I work with a lot of developers taking finished websites and deploying them. When the developer codes, he or she uses what's comfortable to them or what works best with their department. After a number of times trying to...
Integrating Medical Device Product Development with the Quality Management System
A critical business challenge for medical device manufacturers as they scale is getting products to market quickly while supporting existing products and meeting FDA Quality System Regulation (21-CFR-820) requirements. To achieve this effectively, Product Development...
Continuous Integration in 8 Easy Steps with Buildbot
Several weeks ago, Ron provided some great insight regarding how to install the Hudson continuous integration tool on Windows. This week, I'll be discussing a different tool: Buildbot. My initial exposure to continuous integration tools in general was only a few years...