Put it in the cloud. You’ve heard this catch phrase over and over again, more so in recent years with the proliferation of online technologies and services. As more and more PLM software vendors choose to move their application to the cloud, should you consider moving...
product lifecycle management
Automatic Processing of Content on SolidWorks Workgroup PDM Server
One of the powerful features of a full enterprise product lifecycle management (PLM) system is being able to post process content uploaded to it. For example, adding a watermark, or changing a revision of a document automatically. The idea behind Solidworks Workgroup...
Medical Device Interoperability: A $30B opportunity?
Greater medical device interoperability and the adoption of commonly accepted standards could save the US in excess of $30B, suggests a West Health Institute report published in March. Lack of device interoperability creates significant waste and risk to patient...
PLM in the Cloud: Computer System Validation in FDA Regulated Industries
Product lifecycle management (PLM) systems have evolved from being custom-built, on-premise applications to cloud-based, off-the-shelf solutions. As adoption for PLM in the cloud increases, system validation approaches in FDA/GXP regulated industries have had to...
ROHS 2 for Medical Devices: Are You Ready?
As of July 22, 2014, the RoHS (Restriction of Hazardous Substances) Directive must be observed for first time distribution of all medical devices to the full extent. Furthermore, all products with a CE marking must also be RoHS-compliant. ROHS 2 Compliance Changes at...
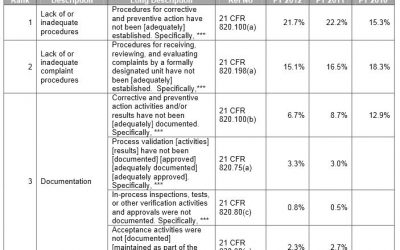
FDA Form 483: Top Ten Observations for Medical Devices
Medical Device manufacturers regulated by the FDA are subject to cGMP (Current Good Manufacturing Practice) regulations and may be inspected by the FDA to ensure compliance. If the FDA inspector(s) observes conditions that in their judgment may constitute violations,...
Integrating Medical Device Product Development with the Quality Management System
A critical business challenge for medical device manufacturers as they scale is getting products to market quickly while supporting existing products and meeting FDA Quality System Regulation (21-CFR-820) requirements. To achieve this effectively, Product Development...