Effectively managing software licenses can be challenging, but it is critical in ensuring your organization works cost-efficiently. These challenges only increase when vendors make unexpected changes. This was precisely the case when Klocwork, a leading static code...
Compliance & Regulatory
Accelerating Medical Software Compliance and Efficiency with SPK ACEs
Compliance with regulatory standards is the number one priority for every medical manufacturer. Ensuring pipelines are secure and compliant doesn’t just ensure safety, but it results in better quality products. When a startup medical manufacturing company contacted...
The FDA UDI Rule: 5 Things You Need to Know
The release of the FDA final rule on Unique Device Identification (UDI) is expected this summer. Here are five things you need to know: 1. What is the UDI Rule? In July 2012, the FDA proposed a rule requiring medical device manufacturers to label their products with...
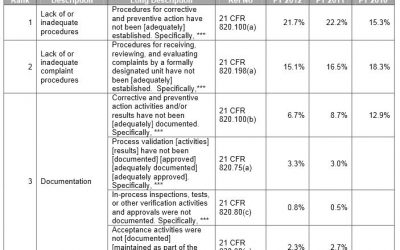
FDA Form 483: Top Ten Observations for Medical Devices
Medical Device manufacturers regulated by the FDA are subject to cGMP (Current Good Manufacturing Practice) regulations and may be inspected by the FDA to ensure compliance. If the FDA inspector(s) observes conditions that in their judgment may constitute violations,...
7 Steps for Implementing ISO 9001 Quality Systems Successfully
Implementing a Quality Management System (QMS) that ensures compliance with ISO 9001 is a strategic decision for organizations. Implementing this system enhances customer satisfaction, improves efficiency, and drives continuous improvement. However, achieving ISO 9001...
How Hybrid Cloud Can Turbo Charge Your Manufacturing Enterprise
Executive Summary That’s the year public and private cloud-based solutions are set to surpass traditional data centers in terms of overall spending. In 2017, nearly two thirds of spending went toward private cloud solutions. Cloud usage exploded in 2017, to the tune...
How AWS Cloud Solutions Can Help Your Manufacturing Enterprise Maintain Its Competitive Edge
Executive Summary In 2020, public and private cloud-based solutions like Amazon Web Services surpassed traditional data centers in terms of overall spending. In 2017, nearly two thirds of spending went toward private cloud solutions. Cloud usage exploded in 2017, to...
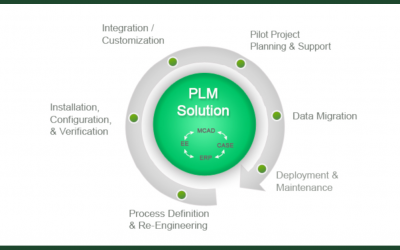
PLM: Automate your Product Development Compliance Process
Developing new and innovative products is essential for companies to survive and thrive -- however safety can never take a backseat to innovation. That is why many companies, like those in the Medical Devices, Aeronautics and Automotive industries, have strict...
How PLM Enables Innovation Without Risking Compliance
As technology has advanced, it's become more complex -- and with complexity comes the issue of tracking and monitoring the different processes and their corresponding data. In non-critical applications, the extent of this reporting is often minimal. However, in...
Top 3 PLM Predictions from Oleg Shilovitsky — Talking Beyond PLM
SPK and Associates co-founder Chris McHale spoke with PLM expert Oleg Shilovitsky, founder of BeyondPLM.com, to get his top three product lifecycle management (PLM) predictions for 2015. PLM vendors will encounter greater complexity when delivering cloud solutions New...
Challenges of Managing a BOM Across the Product Life Cycle (Part 2)
SPK and Associates co-founder Chris McHale spoke with PLM expert Oleg Shilovitsky of BeyondPLM, on the difficulty of BOMs across engineering disciplines. In part two of the discussion, they focus in on the difficulty of BOMs across the product life cycle.
SOTIF ISO/ PAS 21448 Guide eBook
As autonomous technology outpaces regulatory standards, further focus is needed to ensure the safety of self-driving vehicles. SPK and Associates, in collaboration with PTC SOTIF ISO/PAS 21448, brings you an insightful guide - a comprehensive eBook on the Safety of...