You need more than just an antivirus and security suite to keep your system safe. You need multiple, non-redundant solutions covering various aspects of network security. In our last blog, we discussed the role of whitelisting in keeping your network secure. Now we’re...
Compliance & Regulatory
Security and Compliance – What Your Company Needs to Know Part 1: Whitelisting
Security in the 21st century is a complicated game. The good guys are always playing catch up with the bad guys. Security is particularly important for medtech companies due to their specific compliance needs. Banking and fintech likewise have a high bar for security...
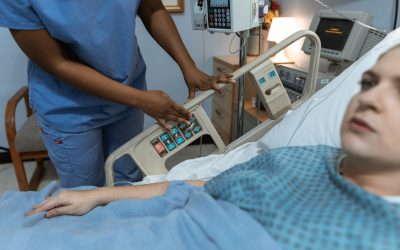
How to Pinpoint the Benefits and Security Risks of Intelligent Medical Devices
Traditional medical devices are quickly going the way of the ear horn for one simple reason: their valuable data is confined to the device. Some have short-term storage capabilities. None, however, capture data over time for long-term analytics or storage. Intelligent...
Podcast: Can IoT Jump These Five Hurdles? Episode 5 – Systems Standards with Built-in Compliance
In episode five of our podcast series "Can IoT Jump These Five Hurdles?", SPK's Director of Strategy, Rajiv Mistry, discusses systems standards and regulation challenges that could impede IoT's future growth and effectiveness. Think VCR v.s. Betamax or Blu-Ray v.s....
Podcast: Can IoT Jump These Five Hurdles? Episode 2 – Sensor Interfaces and Smart Devices
In the second episode of our podcast series "Can IoT Jump These Five Hurdles?", we transition from a broad introduction of these five hurdles to a deeper dive into each challenge facing the Internet of Things. Let's begin with the smart device itself and it's digital...
Risk Management in the PTC Integrity Medical Device Solution
An important part of creating any product intended for use in the medical field is that the manufacturer should have identified all of the risks involved in using the device, and have done their best to mitigate those risks before their product ever sees a patient....
Agile Development in Regulated Environments – Part 1: Yes, it can work
The value system and practices that embody Agile Software Development have been around for well over a decade, and have been touted as having "crossed the chasm" by organizations such as the Agile Alliance, Gartner, and Forrester Research. Numerous studies indicate...
Tackling Email Archiving Regulations
Whether or not your in a heavily regulated industry, having a solid email archiving solution is an absolute necessity. Email’s importance in the workplace for the past decade-and-a-half is similar to the need of having a dial tone in years past. In addition, email is...
PLM in the Cloud: Computer System Validation in FDA Regulated Industries
Product lifecycle management (PLM) systems have evolved from being custom-built, on-premise applications to cloud-based, off-the-shelf solutions. As adoption for PLM in the cloud increases, system validation approaches in FDA/GXP regulated industries have had to...
Blog: Leveraging PTC’s Integrity Platform for IEC 62304 Compliance
SPK and Associates leverage PTC’s Integrity platform to help Medical Device companies develop software efficiently while achieving IEC 62304 compliance.
A Review of FDA 483 Observations – Top Med Device Issues Sited & Proper Response
This article reviews what an FDA 483 Observation looks like, some of the more common issues flagged in medical device companies, and how to respond.
Design Output: A Review of 21 C.F.R. §820.30(d) and FDA Warning Letter Trends
Design Output: A Review of 21 C.F.R. §820.30(d) and FDA Warning Letter Trends